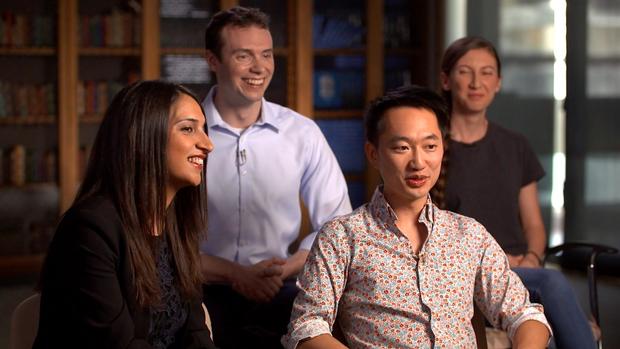Factor X have we finally found the fountain of Youth?
Re: Factor X have we finally found the fountain of Youth?
That's the point. The epigenetic changes are clearly the cause of all of the aging based dysfunction. That's why if someone comes up with a workable cellular reprogramming protocol that both works and is safe, I will most certainly do it. In the meantime, there is mitochondrial related stuff as well as senolytics that I will do starting early next year.
If nuclear DNA really is a cause of aging, there's a fix for that in the form of a CRISPR therapy that is targeted specific to stem cells that was just developed by Harvard. I think it is also this same thing that George Church used to rejuvenate his lab rats a few months ago.
If nuclear DNA really is a cause of aging, there's a fix for that in the form of a CRISPR therapy that is targeted specific to stem cells that was just developed by Harvard. I think it is also this same thing that George Church used to rejuvenate his lab rats a few months ago.
Re: Factor X have we finally found the fountain of Youth?
kurt9 wrote:That's the point. The epigenetic changes are clearly the cause of all of the aging based dysfunction. That's why if someone comes up with a workable cellular reprogramming protocol that both works and is safe, I will most certainly do it. In the meantime, there is mitochondrial related stuff as well as senolytics that I will do starting early next year.
There could be the programmed "epigenetic changes" causing "aging based dysfunction" and also in addition accumulating damage as a result of simply living. Drinking, smoking, breathing polluted air, other contaminates in the environment from food/drink etc. Even if our repair mechanism(s) didn't degrade as a result of epigenetic changes there would still be cumulative damage from that. Merely the fact that we need oxygen to process our food generates free radicals that yes damage us over time even with repair mechanism functioning at the "youthful" level. I am not as ambitious (brave) as you are; my current regime is notable for Elysium Basis (recently) and Longevinex for many years; the later recently added Fisetin to it's ingredients list probably in too low a concentration. Very recently added Berberine to my mix as a substitute for Metformin requiring a prescription. Stem cell therapies are an interest of mine as well but haven't been bold enough (or rich enough) to take advantage of them yet. I have diagnosed arthritis in my left shoulder with accompanying bone spurs. Understand famously that Mel Gibson's then 92 yr old Dad received stem cell therapy for a host of issues with apparently (continuing) good results several years ago.
ADDENDUM; More details on Riordan's treatment regime and facility:Host Michael Beattie discusses stem cell therapy using *human umbilical cord tissue-derived mesenchymal stem cells (hUCT-MSCs) at the Stem Cell Institute in Panama with renowned applied stem cell scientist and founder, Neil Riordan, Pa, PhD and acclaimed actor, director and producer Mel Gibson.
Dr. Riordan discusses the miraculous progress of a spinal cord injury patient and pilot whose doctors said that he would never walk again. He talks about where the stem cells come from, how they work and why they can treat so many seemingly different conditions. Dr. Riordan explains how umbilical cords, and subsequently, hUCT-MSCs used at the Stem Cell Institute are carefully selected using molecular screening. He also discusses why mesenchymal stem cells from umbilical cords function better than MSCs from adults, especially as they age or if they are suffering from a disease like multiple sclerosis. For example. MSCs from a newborn multiply exponentially compared to MSCs from an older adult.
Mel Gibson discusses his father’s miraculous recovery, literally from his deathbed after receiving hUCT-MSCs at the age of 92. He’s currently 99 and still going strong having been treated in Panama several times since then. Mel also discusses his personal experiences in Panama.
Stem Cells: Their Role in Aging and in the Treatment of Chronic Diseases - Neil Riordan, PA, PhD.
Published on May 16, 2019
Dr. Riordan discusses stem cell therapy at the Stem Cell Institute in Panama City, Panama. He mentions the two main types of adult stem cells but his talk focuses on mesenchymal stem cells including their sources and functions. Then he goes on to discuss specifically the specially selected human umbilical cord tissue-derived MSCs used in Panama. He highlights several clinical trials for spinal cord injury, rheumatoid arthritis, diabetes and cancer inhibition. He shows MSCs growing in culture and discusses 3D culturing. He then goes into cord tissue products being offered in the US including Signature Cord, which he developed. Finally, Dr. Riordan discusses current US law and the new stem cell law in Texas, including where he sees the industry in the US going from here.
http://www.cellmedicine.com
https://www.youtube.com/watch?v=f9O3Lyz8qw0
Re: Factor X have we finally found the fountain of Youth?
More about Neil Riordan:
What I've Learned From Neil Riordan And Why I Still Go to Panama For Stem Cell Treatment
https://www.youtube.com/watch?v=73nGjpfqRdkPublished on Sep 2, 2018
Dr. Riordan is the founder of the Stem Cell Institute in Panama. His clinic was where I first went in October 2008 for treatment that I hoped would help the debilitation and immobility my progressive MS had caused. This man's revolutionary regenerative medical approach changed my life! iLoveMyNewStemCells because all that I have continued to learn during my own stem cell journey. Loved this interview and thought others may benefit from hearing more too ...
Re: Factor X have we finally found the fountain of Youth?
The best stem cell therapies are without the stem cells (e.g. cellular reprogramming). That's why its better to wait another 5 years, if possible, rather than doing stem cell treatments that may or may not work.
Re: Factor X have we finally found the fountain of Youth?
Brent Nally interviews Dr. Aubrey de Grey @ SENS on July 17, 2019
https://www.youtube.com/watch?v=TquJyz7tGfk
Rather long interview of Dr. Aubrey de Grey; rather interesting note, at about 13:30 minutes in the interviewer makes reference to the long awaited Bill Andrews' telomerase therapy's "patient zero". He (the interviewer) made reference to a conversation he had with Liz Parrish where she indicated said patient who is said to have late stage Alzheimer's, had finally received the telomerase therapy
ADDENDUM: In response to a posted question:
Published on Jul 18, 2019
My mission is to drastically improve your life by helping you break bad habits, build and keep new healthy habits to make you the best version of yourself. I read the books and do all the research and share my findings with you!
This video is an interview of Dr. Aubrey de Grey @ SENS on July 17, 2019. My wife, Lauren Nally, was our camerawoman.
https://www.youtube.com/watch?v=TquJyz7tGfk
Rather long interview of Dr. Aubrey de Grey; rather interesting note, at about 13:30 minutes in the interviewer makes reference to the long awaited Bill Andrews' telomerase therapy's "patient zero". He (the interviewer) made reference to a conversation he had with Liz Parrish where she indicated said patient who is said to have late stage Alzheimer's, had finally received the telomerase therapy
ADDENDUM: In response to a posted question:
Reply:"Thanks for the interview Brent Nally. At about 13:30 you make reference to Bill Andrews' long awaited telomerase therapy patient. Any idea when Andrews will have something to say about progress/results of the treatments?"
In one of the previous interviews I recall Bill Andrews said himself that he didn't regard Liz Parrish's treatment (which he indicated that he was not directly associated with) to be as comprehensive as the one he was going to do on this patient."I’ll be at RAADfest October 3-6th 2019 in Las Vegas with Dr. Aubrey de Grey, Dr. Bill Andrews, Liz Parrish and many other longevity experts. I’ll ask Bill & Liz for an update on the telomerase gene therapy patient who has Alzheimer’s. This private patient with Alzheimer’s was the second publicly known person (Liz was the first) to receive this telomerase gene therapy. Stay subscribed to my YouTube channel for this update."
Re: Factor X have we finally found the fountain of Youth?
NEWS · 05 September 2019
First hint that body’s ‘biological age’ can be reversed
In a small trial, drugs seemed to rejuvenate the body’s ‘epigenetic clock’, which tracks a person’s biological age.

A person’s biological age, measured by the epigenetic clock, can lag behind or exceed their chronological ageCredit: Patrick McDermott/Getty
https://www.nature.com/articles/d41586-019-02638-w
First hint that body’s ‘biological age’ can be reversed
In a small trial, drugs seemed to rejuvenate the body’s ‘epigenetic clock’, which tracks a person’s biological age.

A person’s biological age, measured by the epigenetic clock, can lag behind or exceed their chronological ageCredit: Patrick McDermott/Getty
A small clinical study in California has suggested for the first time that it might be possible to reverse the body’s epigenetic clock, which measures a person’s biological age.
For one year, nine healthy volunteers took a cocktail of three common drugs — growth hormone and two diabetes medications — and on average shed 2.5 years of their biological ages, measured by analysing marks on a person’s genomes. The participants’ immune systems also showed signs of rejuvenation.
The results were a surprise even to the trial organizers — but researchers caution that the findings are preliminary because the trial was small and did not include a control arm.
“I’d expected to see slowing down of the clock, but not a reversal,” says geneticist Steve Horvath at the University of California, Los Angeles, who conducted the epigenetic analysis. “That felt kind of futuristic.” The findings were published on 5 September in Aging Cell.
“It may be that there is an effect,” says cell biologist Wolfgang Wagner at the University of Aachen in Germany. “But the results are not rock solid because the study is very small and not well controlled.”
Marks of life
The epigenetic clock relies on the body’s epigenome, which comprises chemical modifications, such as methyl groups, that tag DNA. The pattern of these tags changes during the course of life, and tracks a person’s biological age, which can lag behind or exceed chronological age.
Scientists construct epigenetic clocks by selecting sets of DNA-methylation sites across the genome. In the past few years, Horvath — a pioneer in epigenetic-clock research — has developed some of the most accurate ones.
The latest trial was designed mainly to test whether growth hormone could be used safely in humans to restore tissue in the thymus gland. The gland, which is in the chest between the lungs and the breastbone, is crucial for efficient immune function. White blood cells are produced in bone marrow and then mature inside the thymus, where they become specialized T cells that help the body to fight infections and cancers. But the gland starts to shrink after puberty and increasingly becomes clogged with fat.
Evidence from animal and some human studies shows that growth hormone stimulates regeneration of the thymus. But this hormone can also promote diabetes, so the trial included two widely used anti-diabetic drugs, dehydroepiandrosterone (DHEA) and metformin, in the treatment cocktail.
The Thymus Regeneration, Immunorestoration and Insulin Mitigation (TRIIM) trial tested 9 white men between 51 and 65 years of age. It was led by immunologist Gregory Fahy, the chief scientific officer and co-founder of Intervene Immune in Los Angeles, and was approved by the US Food and Drug Administration in May 2015. It began a few months later at Stanford Medical Center in Palo Alto, California.
Fahy’s fascination with the thymus goes back to 1986, when he read a study in which scientists transplanted growth-hormone-secreting cells into rats, apparently rejuvenating their immune systems. He was surprised that no one seemed to have followed up on the result with a clinical trial. A decade later, at age 46, he treated himself for a month with growth hormone and DHEA, and found some regeneration of his own thymus.
In the TRIIM trial, the scientists took blood samples from participants during the treatment period. Tests showed that blood-cell count was rejuvenated in each of the participants. The researchers also used magnetic resonance imaging (MRI) to determine the composition of the thymus at the start and end of the study. They found that in seven participants, accumulated fat had been replaced with regenerated thymus tissue.
Rewinding the clock
Checking the effect of the drugs on the participants’ epigenetic clocks was an afterthought. The clinical study had finished when Fahy approached Horvath to conduct an analysis.
Horvath used four different epigenetic clocks to assess each patient’s biological age, and he found significant reversal for each trial participant in all of the tests. “This told me that the biological effect of the treatment was robust,” he says. What’s more, the effect persisted in the six participants who provided a final blood sample six months after stopping the trial, he says.
“Because we could follow the changes within each individual, and because the effect was so very strong in each of them, I am optimistic,” says Horvath.
Researchers are already testing metformin for its potential to protect against common age-related diseases, such as cancer and heart disease. Fahy says that the three drugs in the cocktail might contribute separately to the effect on biological ageing through unique mechanisms. Intervene Immune is planning a larger study that will include people of different age groups and ethnicities, and women.
Regenerating the thymus could be useful in people who have underactive immune systems, including older people, he says. Pneumonia and other infectious diseases are a major cause of death in people older than 70.
Cancer immunologist Sam Palmer at the Herriot-Watt University in Edinburgh says that it is exciting to see the expansion of immune cells in the blood. This “has huge implications not just for infectious disease but also for cancer and ageing in general”.
https://www.nature.com/articles/d41586-019-02638-w
Re: Factor X have we finally found the fountain of Youth?
I personally know the guy behind this (I've known him for 30 years). He did this because he came up with a way to regenerate his thymus gland. This is definitely useful work and we definitely need to regenerate our thymus glands. However, we need to regenerate our lymphatic system as well in order for that regenerated thymus gland to do its job. However, the fact that biological age (as measured by the Horvoth clock) was reversed is significant in its own right.
Re: Factor X have we finally found the fountain of Youth?
Update about George Church's gene therapy research:

How Much Can We Delay Aging? A Gene Therapy Trial Is About to Find Out
by Shelly Fan - Nov 12, 2019
https://singularityhub.com/2019/11/12/h ... -find-out/

How Much Can We Delay Aging? A Gene Therapy Trial Is About to Find Out
by Shelly Fan - Nov 12, 2019
Aging is reversible.
It’s still a somewhat controversial idea in humans. Yet recent attempts at delaying—or even reversing—diseases that pop up with age in animals clearly show that health doesn’t necessarily decline with age. The slip-and-slide into poor health as we age may seem like a natural trajectory, but it’s not inevitable.
In the lab, just a single treatment can often reverse aging’s nagging effects and prolong healthspan. Plenty of these are on the cusp of clinical trials: metformin and other pills, killing off senescent “zombie” cells, cutting calories, increasing exercise, or infusing “youth factors” in the blood from the young. Add to that dozens of “anti-aging” genes already proven effective in various animal models, and we have a cornucopia of ideas to fight age-related health problems—and even potentially increase lifespan.
Now here’s the mind-bender: if individual treatments already work to a degree, what happens when you combine them? How far, exactly, can we reverse the aging clock?
This week, the legendary synthetic biologist Dr. George Church and team at the Wyss Institute at Harvard University took a first step towards cracking the ultimate question of anti-aging research. They combined three gene therapies, each linked to a health problem associated with aging, into a single vaccine-like shot and gave it to ailing mice. The combination treatment reversed diabetes and obesity while improving heart and kidney function—even when those organs had already begun failing.
“If you hit enough specific diseases, you’re getting at the core aging components that are common to all of them,” said Church.
Rather than genetically modifying the mice, the team used a virus to encode genetic material that “fine-tunes” the activity of all three genes, but leaves the genome alone. In this way, the team explained, the combination therapy is far more easily applicable to humans in the long run.
“Everyone wants to stay as healthy as possible for as long as possible,” said study author Dr. Noah Davidsohn, the chief technology officer of Rejuvenate Bio, which he co-founded with Church and study author Dr. Daniel Oliver. “This study is a first step toward reducing the suffering caused by debilitating diseases.”
Last month, Rejuvenate Bio began testing a similar experimental gene therapy in aging dogs prone to heart problems. If all goes well, humans are next.
“Gene therapy gives you a testable therapy at scale in mice. And we can move from mice to dogs and then to humans. We’re focusing on the reversal of age-related diseases so we’ll be more healthy and youthful later in life,” said Church.
The Combo-Shot Dilemma
Fighting aging often feels like a game of whack-a-mole.
Age is one of the largest risk factors for a myriad of diseases—type II diabetes, liver and kidney troubles, high blood pressure, declining cognitive functions. The classic approach is to tackle each individually, but scientists have long dreamed of a single treatment to stall the development of multiple age-related diseases. For something as complex as aging, however, a single gene, protein, drug, or other “silver bullet” seems unlikely. An alternative is to stack multiple treatments into a single dose, or a combination therapy.
That’s the approach the Church team took. It’s a ballsy move. Combination gene therapies are incredibly rare, partly because they’re the equivalent to sharp-shooting multiple moving targets from a moving train and hitting the bulls-eye every single time. Miss a target—boom, nasty side effects. Hit a target too strong—wham, other unpredictable side effects. Although the idea is conceptually simple, most scientists deem it clinically impractical.
Meet the Players
The Church group went blue sky and focused on three genes well-linked to type two diabetes, heart disease, and kidney failure. Each was “packaged” into a non-toxic virus with affinity to the liver, where most of the gene products are produced.
The first is fibroblast growth factor 21 (FGF21), which keeps metabolism running smoothly and helps the body maintain its blood sugar levels. The second, alpha-Klotho, is a protein that regulates a cell’s regular activity and provides protection to heart and kidney disease. Finally, TGFbeta1 is a negative health factor that spurs age-related heart overgrowth and immune problems, and here the gene therapy is to inhibit its functions with a soluble protein called sTGFβR2.
All three genes have known roles in various age-associated diseases, the team explained. They wanted to see if changing them together could further boost health status in an additive or synergistic way, or if they actually work against each other in some way.
Immediately, FGF21 seemed to do most of the heavy lifting. Even on its own, the gene therapy reduced obesity in overweight mice, and the effects were slightly greater with the two other gene targets. With just a single shot, FGF21 also reversed type two diabetes in a diabetic mouse model, and when combined with sTGFβR2 helped kidney shrinkage in another model with kidney issues.
sTGFβR2 also worked its magic. Alone, it improved heart functions in mice with heart failure; with either of the other gene therapies, the effects were even larger.
“Collectively, these data show that a single combination therapeutic consisting of…[the two genes]…can successfully treat all four age-related disease at once,” the team concluded.
Curiously, all three gene therapies given together worsened the health outcome of some mice. Further digging found that although individually helpful, FGF21 and alpha-Klotho don’t play nice, exacerbating heart and kidney failure. Why this happens is still unclear, but it’s an important lesson for future combo-therapies: more isn’t always better in anti-aging therapies; what matters is how you stack them.
“This research marks a milestone in being able to effectively treat the many diseases associated with aging, and perhaps could lead to a means of addressing aging itself,” said Church.
From Mice to Dogs
Not everyone is on board with the “anti-aging gene therapy” claim. Because the team didn’t track overall lifespan of the treated mice, “it’s impossible to know whether they actually affected the aging process or not,” said Dr. Matt Kaeberlein at University of Washington, who was not involved in the work.
To Church and colleagues, that point’s moot. The focus isn’t on extending longevity, but rather the number of years an aged individual is in good health. Targeting age-related diseases also makes the therapies more attractive for FDA approval, since the agency doesn’t yet consider aging itself a treatable disease. What’s more important, they concede, is understanding how each component contributes to reversing various disease trajectories—who’s doing most of the work, and why combos sometimes exacerbate existing conditions.
The team is already collaborating with the American Cavalier King Charles Spaniel Club, to test the effect of FGF21 and sTGFβR2 in a small trial of 10 dogs. By age eight, these dogs often develop severe heart conditions that shorten their lifespan, and the team hopes the gene therapy can ward off the disease. If successful, they’ll gear up for larger trials that include additional breeds with other age-related health problems.
Although the study focused on only 3, Chruch has at least 45 potential anti-aging gene targets up his sleeve. Especially notable are those linked to neurodegeneration and memory loss, which the lab hopes to try in the near future.
What’s already clear is that scientists don’t have to fear combination gene therapies. “We have … demonstrated that individual longevity gene therapies can be easily combined into a single therapeutic mixture,” the team said.
There’s still a long road ahead before we can go into a drug store and get a longevity vaccine. According to Church’s estimate, the trial in dogs will likely take two years, and even if all goes well, a marketed product won’t be available for more than a decade.
But the study represents only the opening salvo of anti-aging gene therapy. We’re on the cusp of finally answering how much we can turn back the aging clock. The main thing is to get good at reversing age first, said Church. And if that truly works, there’s no upper limit to how long we’ll be able to extend healthy lives one day.
https://singularityhub.com/2019/11/12/h ... -find-out/
Re: Factor X have we finally found the fountain of Youth?
More on Epigenic reprogramming:
Pulsed Yamanaka Factors Set Back Epigenic Age
In a column last month, I posed the question whether the methylation clocks of Horvath are drivers of aging or responses to aging. If we intervene so as to set back the clock, are we signaling the body to be younger, or are we shutting down the repair mechanisms that the body has engaged in response to the damage of aging?
There’s a preprint from David Sinclair’s Harvard laboratory, posted on BioRxiv but not yet published, with very encouraging news for those of us who think that resetting the epigenetic (methylation) clock is a path to anti-aging. They suggest that 3 of the 4 Yamanaka factors, administered in short pulses, can set back the Horvath methylation clock without turning functioning tissues back into stem cells. The same study offers evidence to support the hypothesis that the epigenetic clock is a lethal driver of aging, rather than an adaptive response to damage.

Sinclair opens the paper with an un-footnoted statement that aging consists in accumulated damage, as if this is uncontested and incontrovertible. He refers to the straight-line methylation changes that happen predictably and consistently with age as “epigenetic drift”, as if these changes were random. He believes that they are ‘loss of information” when these changes show every sign of being predictable and directed.
In the standard evolutionary paradigm, the mouse is evolved to live as long as possible, all other things being equal. (To be explicit: I don’t believe this; I think the mouse is evolved for a lifespan optimized to its ecology, not longer or shorter.) If you believe this standard paradigm, then why doesn’t the old mouse reset its epigenetic clock without our having to do it for him? In Sinclair’s account, the mouse has lost information, and can’t do it. But the Yamanaka factors are all in the mouse genome, and if that is all the information the mouse needs, we have to ask why the mouse needs us to send the signals.
We wondered whether mammalian cells might retain a faithful copy of epigenetic information from earlier in life, analogous to Shannon’s “observer” system in Information Theory, essentially a back-up copy of the original signal to allow for its reconstitution at the receiving end if information is lost or noise is introduced during transmission17.
It’s cute that Sinclair invokes Claude Shannon’s foundational theory from the 1930s on transmission errors and signal correction. But is it relevant? The reason that Sinclair and many others assume the information (how to be a young mouse) is lost is that they believe that evolution has motivated the mouse to stay young and keep making babies if only it could. If the information isn’t lost, doesn’t that defeat the very premise of Sinclair’s “lost information” theory of aging?
The point is that Sinclair is a superb experimentalist. He is also realistic enough to accept the overwhelming evidence that aging is an epigenetic program, and that the best way to influence it is to reset our epigenetics. But he is still mired in the old theory that denies it is possible for an aging program to evolve, so his efforts to frame his work in the context of “lost information” and “random drift” are strained to say the least.
Now that I’ve got that off my chest, let’s get on to the substance of this new finding, and the carefully-designed experiments that support these findings. He and co-authors demonstrate that mice treated with OSK (the first 3 out of the 4 Yamanaka factors OSKM) have restored capacity to regenerate damaged nerve cells, a capacity which is normally lost early in life. They go on to show that OSK isn’t directly responsible for regenerative capacity. And they demonstrate that resetting the methylation pattern on the mouse DNA is necessary for the restoration.
Specifically, they engineer mice with a cellular switch that can turn on OSK in response to a applied antibiotics. They flip the switch in the eyes only, then crush the optic nerve to see if it grows back. Normally, a mouse is able to regenerate nerves only while it is in early stages of development.
Yes, the nerves grow back if the eyes are pre-treated with pulsed OSK. And the benefit is lost in the absence of methyl transferase enzymes. This last result was part of the experiment in order to demonstrate that the mechanism for restoration involves re-programming methylation patterns on the chromosomes.

—rDNA methylation age of 12-month-old RGCs FACS isolated from retinas infected for 4 weeks with -OSK or +OSK AAV together with short-hairpin DNAs with a scrambled sequence (sh-Scr) or targeted to Tet1 or Tet2 (sh-Tet1/sh-Tet2).
Questions not addressed yet
I’m inclined to interpret this article as much for what it doesn’t report as for what it does.
In the main experiment, OSK was induced just in the eyes, so it was just the eyes that were rejuvenated. But they also report a “safety” test done, in which OSK was induced in the whole body at a low level for an entire year without toxic effects. Of course, it’s nice to know that the low-dose OSK was not toxic and that cancer risk did not increase. But did the mice benefit from the whole-body treatment? Did they show any signs of rejuvenation, or of enhanced stem cell function?
There is a Horvath methylation clock for mice. Did the mice get younger according to the Horvath clock? The authors report that damaging the retinal nerve made the nerve cells older according to the methylation clock, and that the application of OSK brought the cells back. But I don’t see anywhere in the paper a measurement of the eye’s methylation age before and after the OSK treatment, independent of injury. For that matter, there is no discussion of the methylation age of the mice treated with whole-body OSK for a year. These omissions are curious. Are they suspicious? Have they tried and failed to set back the methylation clock, and they don’t want to report it? Certainly it’s a question I would ask if I were reviewing this ms. Maybe we’ll know the answer when the paper is published.
Did mice live longer after treatment with OSK? Answering this one takes time, and perhaps the Sinclair lab has mice even now that are living longer, but it will be a few years before we know. Or perhaps the treatment has failed so far to extend lifespan, and Sinclair is reluctant to report a failure.
https://joshmitteldorf.scienceblog.com/ ... genic-age/
Pulsed Yamanaka Factors Set Back Epigenic Age
In a column last month, I posed the question whether the methylation clocks of Horvath are drivers of aging or responses to aging. If we intervene so as to set back the clock, are we signaling the body to be younger, or are we shutting down the repair mechanisms that the body has engaged in response to the damage of aging?
There’s a preprint from David Sinclair’s Harvard laboratory, posted on BioRxiv but not yet published, with very encouraging news for those of us who think that resetting the epigenetic (methylation) clock is a path to anti-aging. They suggest that 3 of the 4 Yamanaka factors, administered in short pulses, can set back the Horvath methylation clock without turning functioning tissues back into stem cells. The same study offers evidence to support the hypothesis that the epigenetic clock is a lethal driver of aging, rather than an adaptive response to damage.

Sinclair opens the paper with an un-footnoted statement that aging consists in accumulated damage, as if this is uncontested and incontrovertible. He refers to the straight-line methylation changes that happen predictably and consistently with age as “epigenetic drift”, as if these changes were random. He believes that they are ‘loss of information” when these changes show every sign of being predictable and directed.
In the standard evolutionary paradigm, the mouse is evolved to live as long as possible, all other things being equal. (To be explicit: I don’t believe this; I think the mouse is evolved for a lifespan optimized to its ecology, not longer or shorter.) If you believe this standard paradigm, then why doesn’t the old mouse reset its epigenetic clock without our having to do it for him? In Sinclair’s account, the mouse has lost information, and can’t do it. But the Yamanaka factors are all in the mouse genome, and if that is all the information the mouse needs, we have to ask why the mouse needs us to send the signals.
We wondered whether mammalian cells might retain a faithful copy of epigenetic information from earlier in life, analogous to Shannon’s “observer” system in Information Theory, essentially a back-up copy of the original signal to allow for its reconstitution at the receiving end if information is lost or noise is introduced during transmission17.
It’s cute that Sinclair invokes Claude Shannon’s foundational theory from the 1930s on transmission errors and signal correction. But is it relevant? The reason that Sinclair and many others assume the information (how to be a young mouse) is lost is that they believe that evolution has motivated the mouse to stay young and keep making babies if only it could. If the information isn’t lost, doesn’t that defeat the very premise of Sinclair’s “lost information” theory of aging?
The point is that Sinclair is a superb experimentalist. He is also realistic enough to accept the overwhelming evidence that aging is an epigenetic program, and that the best way to influence it is to reset our epigenetics. But he is still mired in the old theory that denies it is possible for an aging program to evolve, so his efforts to frame his work in the context of “lost information” and “random drift” are strained to say the least.
Now that I’ve got that off my chest, let’s get on to the substance of this new finding, and the carefully-designed experiments that support these findings. He and co-authors demonstrate that mice treated with OSK (the first 3 out of the 4 Yamanaka factors OSKM) have restored capacity to regenerate damaged nerve cells, a capacity which is normally lost early in life. They go on to show that OSK isn’t directly responsible for regenerative capacity. And they demonstrate that resetting the methylation pattern on the mouse DNA is necessary for the restoration.
Specifically, they engineer mice with a cellular switch that can turn on OSK in response to a applied antibiotics. They flip the switch in the eyes only, then crush the optic nerve to see if it grows back. Normally, a mouse is able to regenerate nerves only while it is in early stages of development.
Yes, the nerves grow back if the eyes are pre-treated with pulsed OSK. And the benefit is lost in the absence of methyl transferase enzymes. This last result was part of the experiment in order to demonstrate that the mechanism for restoration involves re-programming methylation patterns on the chromosomes.

—rDNA methylation age of 12-month-old RGCs FACS isolated from retinas infected for 4 weeks with -OSK or +OSK AAV together with short-hairpin DNAs with a scrambled sequence (sh-Scr) or targeted to Tet1 or Tet2 (sh-Tet1/sh-Tet2).
Questions not addressed yet
I’m inclined to interpret this article as much for what it doesn’t report as for what it does.
In the main experiment, OSK was induced just in the eyes, so it was just the eyes that were rejuvenated. But they also report a “safety” test done, in which OSK was induced in the whole body at a low level for an entire year without toxic effects. Of course, it’s nice to know that the low-dose OSK was not toxic and that cancer risk did not increase. But did the mice benefit from the whole-body treatment? Did they show any signs of rejuvenation, or of enhanced stem cell function?
There is a Horvath methylation clock for mice. Did the mice get younger according to the Horvath clock? The authors report that damaging the retinal nerve made the nerve cells older according to the methylation clock, and that the application of OSK brought the cells back. But I don’t see anywhere in the paper a measurement of the eye’s methylation age before and after the OSK treatment, independent of injury. For that matter, there is no discussion of the methylation age of the mice treated with whole-body OSK for a year. These omissions are curious. Are they suspicious? Have they tried and failed to set back the methylation clock, and they don’t want to report it? Certainly it’s a question I would ask if I were reviewing this ms. Maybe we’ll know the answer when the paper is published.
Did mice live longer after treatment with OSK? Answering this one takes time, and perhaps the Sinclair lab has mice even now that are living longer, but it will be a few years before we know. Or perhaps the treatment has failed so far to extend lifespan, and Sinclair is reluctant to report a failure.
https://joshmitteldorf.scienceblog.com/ ... genic-age/
Re: Factor X have we finally found the fountain of Youth?
A Harvard geneticist's goal: to protect humans from viruses, genetic diseases, and aging
George Church's lab at Harvard Medical School is working to make humans immune to all viruses, eliminate genetic diseases and reverse the aging process. Scott Pelley reports on how close the geneticist's team is to a breakthrough.
Dec 08 Correspondent
Scott Pelley
George Church's lab at Harvard Medical School is working to make humans immune to all viruses, eliminate genetic diseases and reverse the aging process. Scott Pelley reports on how close the geneticist's team is to a breakthrough.
Dec 08 Correspondent
Scott Pelley
https://www.cbsnews.com/news/harvard-ge ... 019-12-08/Our lives have been transformed by the information age. But what's coming next is likely to be more profound, call it the genetic information age. We have mapped the human genome and in just the last few years we have learned to read and write DNA like software. And you're about to see a few breakthroughs-in-waiting that would transform human health. For a preview of this revolution in evolution we met George Church, a world leading geneticist, whose own DNA harbors many eccentricities and a few genes for genius.
George Church on using Jeffrey Epstein money
The complicated ethics of genetic engineering
We found George Church in here.
Cory Smith: Most of these are frozen George. Little bits of George that we have edited all in different tubes.
Church threw himself into his work, literally. His DNA is in many of the experiments in his lab at Harvard Medical School. The fully assembled George Church is 6'5" and 65. He helped pioneer mapping the human genome and editing DNA. Today, his lab is working to make humans immune to all viruses, eliminate genetic diseases, and reverse the effects of time.
Scott Pelley: One of the things your lab is working on is reversing aging.
George Church: That's right.
Scott Pelley: How is that possible?
George Church: Reversing aging is one of these things that is easy to dismiss to say either we don't need it or is impossible or both.
Scott Pelley: Oh, we need it.
George Church: Okay. We need it. That's good. We can agree on that. Well, aging reversal is something that's been proven about eight different ways in animals where you can get, you know, faster reaction times or, you know, cognitive or repair of damaged tissues.
Scott Pelley: Proven eight different ways. Why isn't this available?
George Church: It is available to mice.
In lucky mice, Church's lab added multiple genes that improved heart and kidney function and levels of blood sugar. Now he's trying it in spaniels.
Scott Pelley: So is this gene editing to achieve age reversal?
George Church: This is adding genes. So, it's not really editing genes. It's, the gene function is going down, and so we're boosting it back up by putting in extra copies of the genes.
Scott Pelley: What's the time horizon on age reversal in humans?
George Church: That's in clinical trials right now in dogs. And so, that veterinary product might be a couple years away and then that takes another ten years to get through the human clinical trials.
Human trials of a personal kind made George Church an unlikely candidate to alter human evolution. Growing up in Florida, Church was dyslexic, with attention deficit, and frequently knocked out by narcolepsy.
Scott Pelley: What was it that made you imagine that you could be a scientist?
George Church: The thing that got me hooked was probably the New York World's Fair in 1964. I thought this is the way we should all be living. When I went back to Florida, I said, "I've been robbed," you know? "Where is it all?" So, I said, "Well, if they're not going to provide it, then I'm gonna provide it for myself."
With work and repetition, he beat his disabilities and developed a genius for crystallography, a daunting technique that renders 3D images of molecules through X-rays and math. But in graduate school at Duke, at the age of 20, his mania for the basic structures of life didn't leave time for the basic structure of life.
Scott Pelley: You were homeless for a time.
George Church: Yeah. Briefly.
Scott Pelley: Six months.
George Church: Six months.
Scott Pelley: And where were you sleeping when you were homeless?
George Church: Well, yeah. I wasn't sleeping that much. I was mostly working. I'm narcoleptic. So, I fall asleep sitting up anyway.
His devotion to crystallography was his undoing at Duke.
George Church: I was extremely excited about the research I was doing. And so, I would put in 100-plus hours a week on research and then pretty much didn't do anything else.
Scott Pelley: Not go to class.
George Church: I wouldn't go to class. Yeah.
Duke kicked him out with this letter wishing him well in a field other than biology. But, it turned out, Harvard needed a crystallographer. George Church has been here nearly 40 years. He employs around 100 scientists, about half-and-half men and women.
Scott Pelley: Who do you hire?
George Church: I hire people that are self-selecting, they see our beacon from a distance away. There are a lot of people that are a little, you know, might be considered a little odd. "Neuroatypicals," some of us are called.
Scott Pelley: "Neuroatypical?"
George Church: Right.
Scott Pelley: Unusual brains?
George Church: Right, yeah.
Parastoo Khoshakhlagh: One thing about George that is very significant is that he sees what you can't even see in yourself.
Parastoo Khoshakhlagh and Alex Ng are among the "neuroatypicals." They're engineering human organ tissue.
Cory Smith: I think he tries to promote no fear of failure. The only fear is not to try at all.
Cory Smith's project sped up DNA editing from altering three genes at a time to 13,000 at a time. Eriona Hysolli went to Siberia with Church to extract DNA from the bones of wooly mammoths. She's editing the genes into elephant DNA to bring the mammoth back from extinction.
Eriona Hysolli: We are laying the foundations, perhaps, of de-extinction projects to come.
Scott Pelley: De-extinction.
Eriona Hysolli: Yes.
Scott Pelley: I'm not sure that's a word in the dictionary yet.
Eriona Hysolli: Well, if it isn't, it should be.
Scott Pelley: You know there are people watching this interview who think that is playing God.
George Church: Well, it's playing engineer. I mean, humans have been playing engineer since the dawn of time.
Scott Pelley: The point is, some people believe that you're mucking about in things that shouldn't be disturbed.
George Church: I completely agree that we need to be very cautious. And the more powerful, or the more rapidly-moving the technology, the more cautious we need to be, the bigger the conversation involving lots of different disciplines, religion, ethics, government, art, and so forth. And to see what it's unintended consequences might be.
Church anticipates consequences with a full time ethicist in the lab and he spends a good deal of time thinking about genetic equity. Believing that genetic technology must be available to all, not just those who can afford it.
We saw one of those technologies in the hands of Alex Ng and Parastoo Khoshakhlagh. They showed us what they call "mini-brains," tiny dots with millions of cells each. They've proven that cells from a patient can be grown into any organ tissue, in a matter of days, so drugs can be tested on that patient's unique genome.
Scott Pelley: You said that you got these cells from George's skin? How does that work?
Alex Ng: We have a way to reprogram essentially, skin cells, back into a stem cell state. And we have technologies where now we can differentiate them into tissue such as brain tissue.
Scott Pelley: So you went from George's skin cells, turned those into stem cells, and turned those into brain cells.
Alex Ng: Exactly. Exactly.
Scott Pelley: Simple as that.
Organs grown from a patient's own cells would eliminate the problem of rejection. Their goal is to prove the concept by growing full sized organs from Church's DNA.
George Church: It's considered more ethical for students to do experiments on their boss than vice versa and it's good to do it on me rather than some stranger because I'm as up to speed as you can be on the on the risks and the benefits. I'm properly consented. And I'm unlikely to change my mind.
Alex Ng: We have a joke in the lab, I mean, at some point, soon probably, we're going to have more of his cells outside of his body than he has himself.
Church's DNA is also used in experiments designed to make humans immune to all viruses.
George Church: We have a strategy by which we can make any cell or any organism resistant to all viruses by changing the genetic code. So if you change that code enough you now get something that is resistant to all viruses including viruses you never characterized before.
Scott Pelley: Because the viruses don't recognize it anymore?
George Church: They expect a certain code provided by the host that they replicate in. the virus would have to change so many parts of its DNA or RNA so that it can't change them all at once. So, it's not only dead. But it can't mutate to a new place where it could survive in a new host.
Yes, he's talking about the cure for the common cold and the end of waiting for organ transplants. It's long been known that pig organs could function in humans. Pig heart valves are routinely transplanted already. But pig viruses have kept surgeons from transplanting whole organs. Church's lab altered pig DNA and knocked out 62 pig viruses.
Scott Pelley: What organs might be transplanted from a pig to a human?
George Church: Heart, lung, kidney, liver, intestines, various parts of the eye, skin. All these things.
Scott Pelley: What's the time horizon on transplanting pig organs into human beings?
George Church: you know, two to five years to get into clinical trials. And then again it could take ten years to get through the clinical trials.
Church is a role model for the next generation. He has co-founded more than 35 startups. Recently, investors put $100 million into the pig organ work. Another Church startup is a dating app that compares DNA and screens out matches that would result in a child with an inherited disease.
George Church: You wouldn't find out who you're not compatible with. You'll just find out who you are compatible with.
Scott Pelley: You're suggesting that if everyone has their genome sequenced and the correct matches are made, that all of these diseases could be eliminated?
George Church: Right. It's 7,000 diseases. It's about 5% of the population. It's about a trillion dollars a year, worldwide.
Church sees one of his own genetic differences as an advantage. Narcolepsy lulls him several times a day. But he wakes, still in the conversation, often, discovering inspiration in his twilight zone.
Scott Pelley: If somebody had sequenced your genome some years ago, you might not have made the grade in some way.
George Church: I mean, that's true. I would hope that society sees the benefit of diversity not just ancestral diversity, but in our abilities. There's no perfect person.
Despite imperfection, Church has co-authored 527 scientific papers and holds more than 50 patents. Proof that great minds do not think alike.
The best science can tell, it was about 4 billion years ago that self-replicating molecules set off the spark of biology. Now, humans hold the tools of evolution, but George Church remains in awe of the original mystery: how chemistry became life.
Scott Pelley: Is the most amazing thing about life, then, that it happened at all?
George Church: It is amazing in our current state of ignorance. We don't even know if it ever happened ever in the rest of the universe. it's awe-inspiring to know that it either happened billions of times, or it never happened. Both of those are mind boggling. It's amazing that you can have such complex structures that make copies of themselves. But it's very hard to do that with machines that we've built. So, we're engineers. But we're rather poor engineers compared to the pseudo engineering that is biological evolution.
-
paperburn1
- Posts: 2494
- Joined: Fri Jun 19, 2009 5:53 am
- Location: Third rock from the sun.
Re: Factor X have we finally found the fountain of Youth?
I am not a nuclear physicist, but play one on the internet.
Re: Factor X have we finally found the fountain of Youth?
Underdog Pharma Could Reverse Cardiovascular Disease Which is the Leading Medical Problem
Brian Wang | January 13, 2020

Brian Wang | January 13, 2020

https://www.nextbigfuture.com/2020/01/u ... oblem.htmlUnderdog Pharma is developing disease-modifying treatments for atherosclerosis and other age-related diseases.
They want to prevent or reverse atherosclerosis by removing a harmful lipid known as 7-ketocholesterol (7KC) from the arterial walls.
Underdog has a molecule that can extract the oxidized waste from the body. It is a variant of cyclodextrin which is an existing drug that is already approved by the FDA and has a good safety profile.
They will take a classic pharmaceutical approach and use it to attack the root causes of cardiovascular disease. If this works they will be able to reverse heart and arterial disease.
About 610,000 people die of heart disease in the United States every year–that’s 1 in every 4 deaths. Heart disease is the leading cause of death for both men and women.
Cardiovascular disease (CVD), listed as the underlying cause of death, accounted for 840,678 deaths in the US in 2016, approximately 1 of every 3 deaths.
Cardiovascular diseases claim more lives each year than all forms of cancer and Chronic Lower Respiratory Disease combined.
Between 2013 and 2016, 121.5 million American adults had some form of cardiovascular disease.
Between 2014 and 2015, direct and indirect costs of total cardiovascular diseases and stroke were $351.2 billion ($213.8 billion in direct costs and $137.4 billion in lost productivity/mortality).
Cardiovascular disease is the leading global cause of death, accounting for more than 17.6 million deaths per year in 2016, a number that is expected to grow to more than 23.6 million by 2030, according to a 2014 study.
CVD and stroke accounted for 14% of total health expenditures in 2014-2015. This is more than any major diagnostic group.
Total direct medical costs of CVD are projected to increase to $749 billion in 2035, according to a 2016 study.
Re: Factor X have we finally found the fountain of Youth?
This could be something; I respect the source but I don't like that they are being very sketchy about the details:
https://joshmitteldorf.scienceblog.com/ ... akthrough/
Age Reduction Breakthrough
If you eschew hyperbole and hang in for the long haul, maintaining a discipline of understatement in the midst of a flashy neon world, you may be offered a modicum of credence when you make an extraordinary announcement. No one is entitled to this courtesy twice. If the news that you trumpet to the moon does not pan out, your readers will be justified in discounting everything you say thereafter.
Here goes.
I believe major rejuvenation has been achieved in a mammal, using a relatively benign intervention that shows promise of scaling up to humans. I’m going to stake my reputation on it.
—Cartoon by Maddy Ballard
In the race to effect substantial, system-wide rejuvenation, Harold Katcher is a dark horse. He has the right academic credentials and a solid history of research. In fact, in earlier life he was part of a team that discovered the breast cancer gene, brca1. I asked Harold for a biographical sketch, and have printed it in a box at the end of this posting.
But Katcher has no research grants or university lab or venture capital funding, no team of grad students mining databases and screening chemicals in the back room.
One thing Katcher has going for him is the correct theory. Most of the explosion in aging research (and virtually all the venture capital startups) are looking to treat aging at the cellular level. Their paradigm is that aging is an accumulation of molecular damage, and they see their job as engineering of appropriate repair mechanisms.
The truth, as Katcher understands it, is that, to a large extent, aging is coordinated system-wide via signal molecules in the blood. It was our common realization of this vision that brought Katcher and me together more than a decade ago. Katcher briefly describes his 2009 epiphany below. It was the source of his 2013 essay (it took a few years to get it into print) on the significance of parabiosis experiments for the future of aging science.
Of course, Katcher was not the only one to get the message about the power of signal molecules in the blood to reprogram tissues to a younger state throughout the body. The problem is that there are thousands of constituents represented in tiny concentrations in blood plasma, but conveying messages that cells read. Which of these are responsible for aging? A small number of labs, including the Conboys at Berkeley, Amy Wager at Harvard, and Tony Wyss-Coray at Stanford have been searching for the answer over the last decade and more.
Katcher has been able to guess or intuit or experimentally determine the answer to this question. With seed funding from Akshay Sanghavi, he set up a lab in Mumbai two years ago, and tried to rejuvenate old lab rats, using a fraction extracted from the blood of younger rats. The first round of experiments were encouraging, published in this space a year ago. He obtained the next round of funding from a reader of this blog, and had enough rats to titrate dosages experimentally, and to see if treated rats who aged again over time could be re-treated successfully.
There is a hole in this story that awaits the resolution of intellectual property rights. Katcher and Sanghvi have not applied for patents and have not yet found a suitable partner to provide financing for human trials. They have not revealed any details of the treatment, besides the fact that it is in four intravenous doses, and that it is derived from a fraction of blood plasma. Katcher thinks that the molecules involved will not be difficult to manufacture, so that when a product is eventually commercialized, it will not require extraction from the blood of live subjects, rodent or human.
We’re still waiting for longevity curves of these treated rats. In the meantime, the best available surrogate measure of age comes from methylation clocks, as developed by Steve Horvath at UCLA, and other scientists as well. Crucially, Katcher found an ally in Horvath, who didn’t just test his rejuvenated rats, but did the needed statistical analysis to develop a set of six methylation clocks specialized to rats. FIve of the clocks are optimized for different tissues, and one is calibrated across species, so that it can measure age in humans as well as corresponding age in “rat years” (about 1/40 human year). The two-species clock was a significant innovation, a first bridge for translating results from an animal model into their probable equivalent in humans.
In a paper posted to BioRxiv on Friday, https://www.biorxiv.org/content/10.1101 ... 1.full.pdf Katcher and Horvath report results of the methylation measurements in rejuvenated rats. “Crucially, plasma treatment of the old rats [109 weeks] reduced the epigenetic ages of blood, liver and heart by a very large and significant margin, to levels that are comparable with the young rats [30 weeks]….According to the final version of the epigenetic clocks, the average rejuvenation across four tissues was 54.2%. In other words, the treatment more than halved the epigenetic age.”
—Human-rat clock measure of relative age defined as age/maximum species lifespan.
Besides the methylation clock, the paper presents evidence of rejuvenation by many other measures. For example:
IL-6, a marker of inflammation, was restored to low youthful levels
Glutathione (GSH), superoxide dismutase (SOD), and other anti-oxidants were restored to higher youthful levels
In tests of cognitive function (Barnes maze), treated rats scored better than old rats, but not as well as young rats.
Blood triglycerides were brought down to youthful levels
HDL cholesterol rose to youthful levels
Blood glucose fell toward youthful levels
A major question in blood plasma rejuvenation experiments has been how often the cure must be administered. Many of the components of blood plasma are short-lived, secreted into the blood and absorbed continuously throughout the day. The good news from Katcher’s results is that it seems only four injections are needed in order to achieve rejuvenation.
A second question which these experiments resolve is whether rejuvenation requires both adding and removing molecular species from the blood plasma. For example, pro-inflammatory cytokines are found in old blood at much higher levels. Irina and Mike Conboy, people who I regard as most credible in the field, have said that removing bad actors from the blood is probably more important than restoring youthful levels of beneficial signals. They were grad students at Stanford 15 years ago, when the modern wave of parabiosis science was initiated, and have pursued the subject continuously ever since. Katcher’s experiments have achieved their results only by adding blood components, not by removing or even neutralizing others. This suggests that he has found the necessary formula for re-programming epigenetics, so that lower levels of the bad actors occur as a result. But it remains to be seen whether even better results can be obtained if some plasma constituents are removed.
A question that remains unresolved concerns the location and mechanism of the aging clock. I have been undecided over the years between two models:
1.There is a central aging clock, perhaps in the hypothalamus, which keeps its own time and transmits signals throughout the body that coordinate methylation state of dispersed tissues
2.Information about epigenetic age is dispersed through the body, and the body’s clock is a feedback loop that is continually updating methylation age locally in response to signals received about the methylation age globally.
There is a suggestion in the data that the hypothalamus may be more difficult to rejuvenate than other tissues. Does it play a more important role than other tissues in coordinating the age of the entire body? Horvath (personal communication) counsels caution in drawing this inference until measurements are corroborated and more experiments are done.
The Bottom Line
These results bring together three threads that have been gaining credibility over the last decade. Mutually reinforcing, the three have a strength that none of them could offer separately.
The root cause of aging is epigenetic progression = changes in gene expression over a lifetime.
Methylation patterns in nuclear DNA are not merely a marker of aging, but its primary source. Thus aging can be reversed by reprogramming DNA methylation.
Information about the body’s age state is transmitted system-wide via signal molecules in the blood. Locally, tissues respond to these signals and adopt a young or an old cellular phenotype as they are directed.
Harold Katcher, Biographical Sketch
So, you might consider me a late bloomer. While I have thousands of citations in the literature, with publications ranging from the discovery of the human ‘breast cancer gene’, to protein structure, bacteriology, biotechnology, bioinformatics, and biochemistry, there was no center or direction to my work as I had given up my personal goal of solving/curing aging when I learned that ‘wear and tear’ was the cause of it. Yet something happened in year 1985 when I was in California working with Michael Waterman and Temple Smith (fathers of bioinformatics) that is inexplicable: I found myself in Intensive Care with a tube inserted into my trachea and the knowledge that I might not live. And then I had a dream: I dreamed that somehow in the far future (and on another world), I was being feted for ‘bringing immortality to mankind’. Clearly, I survived that incident (started with an infected tooth). I lived a wonderful life – becoming a computer programmer (which I loved), leaving that for the University of Maryland’s Asian division, becoming a full professor and then the Academic Director for the Sciences, in Tokyo, Japan. By the time I left Japan in 2004, (my daughter Sasha was a fourth-grader, (yonensei), in the Japanese school system), I was teaching for U of M online – somewhat retired, and looking forwards to writing computer programs for fun and profit. Yet I never ever forgot that dream. It was clearly impossible; I had no lab – and really, there was no way to repair all damaged cells – it’d be like sweeping back the ocean. And then, in 2009, I read an old paper from 2005, a paper written by the Conboys, (Michael and Irina), Tom Rando and others, coming from Irv Weisman’s lab, that completely changed my life; that showed me that everything I believed about aging was wrong – that aging occurred at the organismic level, not at the cellular level and could be reversed. Well, the rest of the story is about persistence and the blessed intervention of Akshay Sanghvi who too saw there was another way and provided the structural, monetary, and emotional support (and some good ideas) that had me start a new career at age 72 in Mumbai, India. I feel twenty years younger than I did three years ago, I guess that’s another hint about aging. Now the ‘mystical’ dream? It wouldn’t be the first time in history that that happened – take that as a datum.
https://joshmitteldorf.scienceblog.com/ ... akthrough/
Re: Factor X have we finally found the fountain of Youth?
More related from same source:
Out With the Old Blood
https://joshmitteldorf.scienceblog.com/ ... old-blood/
Out With the Old Blood
There is great promise in 2020 that we might be able to make our bodies young without having to explicitly repair molecular damage, but just by changing the signaling environment.
Do we need to add signals that say “young” or remove signals that say “old”?
Does infusion of biochemical signals from young blood plasma rejuvenate tissues of an old animal? Or are there dissolved signal proteins in old animals that must be removed?
For a decade, Irena and Mike Conboy have been telling us removal of bad actors is more important. But just last month, Harold Katcher reported spectacular success by infusing a plasma fraction while taking away nothing. Then, last week, the Conboys came back with a demonstration of the rejuvenating power of simple dilution. [Link to their new paper]
Dilution procedure
They simply replaced half of the blood plasma in 2-year-old mice with a saline solution containing 5% albumin. What is albumin? Blood plasma is chock full of dissolved proteins, about 10% by weight. About half of these are termed albumin. Albumin is the generic portion. It doesn’t change through the lifetime. It doesn’t carry information by itself. But albumin transports nutrients and minerals through the body.
The Conboys took care to show that albumin has no rejuvenation power on its own, and had nothing to do with their experimental results. Rather, they had to replenish albumin in diluting blood, because the animals would be sickened if half their albumin were removed. Replacing the albumin in a transfusion is akin to replacing the volume of water or maintaining the salinity.
In preparation for this experiment, the Conboys have invested years in miniaturizing the technology for blood transfusions, so that mice can be subjected to the same procedures that are commonplace in human hospitals.
Dose-Response
The Conboy lab replaced 50% of mouse blood plasma. They got spectacular results with a single treatment, based on a lucky guess. They have not yet experimented with 30% or 70%. They don’t know yet how long the treatment will last and how long it needs to be repeated.
Evidence of rejuvenation
As with previous papers from the Conboy lab, the group focused on repair and stem cell activity as evidence of a more youthful state. Three separate tissue samples were taken from liver, muscle, and brain.
“Muscle repair was improved, fibrosis was attenuated, and inhibition of myogenic proliferation was switched to enhancement; liver adiposity and fibrosis were reduced; and hippocampal neurogenesis was increased.”
They measured nerve growth factors in the brain, and detected a more robust response, typical of young mice
They lacerated muscles and showed repair rates typical of much younger animals
They examined microscope slides of liver tissue, and showed that it is less fatty and striated than is typical of older mice
—Figure 2. Rejuvenation of adult myogenesis, and albumin-independent effects of TPE. One day after the NBE, muscle was injured at two sites per TA by cardiotoxin; 5 days later muscle was isolated and cryosectioned at 10 µm. (A) Representative H&E and eMyHC IF images of the injury site. Scale bar = 50 µm. (B) Regenerative index: the number of centrally nucleated myofibers per total nuclei. OO vs.ONBE p = 0.000001, YY vs ONBE non-significant p = 0.4014; Fibrotic index: white devoid of myofibers areas. OO vs ONBE p = 0.000048, YY vs YNBE non-significant p = 0.1712. Minimal Feret diameter of eMyHC+ myofibers is normalized to the mean of YY [9]. OO vs. ONBE p=3.04346E-05, YY vs. YNBE p=0.009. Data-points are TA injury sites of 4-5 YNBE and 5 ONBE animals. Young and Old levels (detailed in Supplementary Figure 1) are dashed lines. Representative images for YY versus YNBE cohorts are shown in Supplementary Figure 6. (C) Automated microscopy quantification of HSA dose response, as fold difference in BrdU+ cells from OPTI-MEM alone (0 HSA). There was no enhancement of myogenic proliferation at 1-16% HSA. N=6. (D) Meta-Express quantification of BrdU+ cells by automated high throughput microscopy for myoblasts cultured with 4% PreTPE versus PostTPE serum and (E) for these cells cultured with 4% of each: PreTPE serum + HSA or PostTPE serum + HSA. Significant increase in BrdU positive cells is detected in every subject 1, 2, 3, and 4 for TPE-treated serum (p=0.011, <0.0001, <0.0001, 0.0039, respectively), as well as for TPE-treated serum when 4%HSA is present (p<0.0001, <0.0001, <0.0001, =0.009 respectively). N=6. (F) Scatter plot with Means and SEM of all Pre-TPE, Post-TPE, +/- HSA cohorts shows significant improvement in proliferation in Pre TPE as compared to and Post TPE cohorts (p*=0.033), as well as Pre+HSA and Post+HSA cohorts (p*=0.0116). In contrast, no significant change was observed when comparing Pre with Pre+HSA (p=0.744) or Post with Post+HSA (p=0.9733). N=4 subjects X 6 independent assays for each, at each condition. (G) Representative BrdU IF and Hoechst staining in sub-regions of one of the 9 sites that were captured by the automated microscopy. Blood serum from old individuals diminished myogenic cell proliferation with very few BrdU+ cells being visible (illustrated by one positive cell in Pre-TPE and arrowhead pointing to the corresponding nucleus); TPE abrogated this inhibition but HSA did not have a discernable effect.
[/i]
What’s missing? They did not test any measures of physical or cognitive performance at the level of the organism.
Evidence of behavioral changes (learning and memory, endurance, strength)
Inflammatory markers
Blood lipids
Methylation clock (Horvath, UCLA) or proteomic clock (Lehallier, Stanford)
Some of this is planned for future research. Mike and Irina plan to submit tissue samples for analysis by the Horvath mouse methylation clock.
Clock?
I am a committed enthusiast for the methylation and proteomic clocks that are the best surrogates we have for aging. These technologies can tell us whether anti-aging interventions have been effective without having to wait for animals (or humans) to die before reporting results. But the Conboys still regard these technologies as unproven, and they bristle at the word “clock”. The closest they come is to catalog the entire proteome of treated mice, comparing it to untreated young and old mice.
Multi-dimensional t-SNE analyses and Heatmapping of these data revealed that the ONBE proteome became significantly different from OO and regained some similarities to the YY proteome. Supplementary Figure 4 confirms the statistical significance of this comparative proteomics through Power Analysis, and shows the YY vs. OO Heatmap, where the age-specific differences are less pronounced than those between OO vs. ONBE, again emphasizing the robust effect of NBE on the molecular composition of the systemic milieu.
Translation: As controls, they had mice that underwent plasma exchange with mice of similar age. YY were young, positive controls, and OO were old, negative controls. Treated mice were ONBE=”Old—Neutral Blood Exchange”. Rather than relying on “clock” algorithms that compute an age from the proteome, they compared the entire proteomes of test animals with those of old and young animals, and foud that they resembled the young animals more closely.
Aging and epigenetics
I was an early advocate of the theory that aging is driven primarily by changes in epigenetics. Other proponents include Johnson, Rando, and Horvath. This theory is now mainstream, though its acceptance is far from universal. (The main reason people have difficulty with the idea is the question, “why would the body evolve to destroy itself?” I present a comprehensive answer in my popular book and my academic book.)
On the face of it, the new Conboy result is powerful evidence for the epigenetic theory. They have shown that there are proteins in the blood that actively retard growth and healing. Remove half theses proteins and the animals are able to grow youthful tissues and to heal better. The obvious conclusion is that, with age, there are signaling changes in the blood that weaken the animal and inhibit repair.
There are, however, other ways to interpret the changes. Aubrey de Grey has said (personal communication)
“When everything in the blood except the cells and the albumin is replaced by water, the body will definitely respond by synthesising and secreting everything that it detects a shortage of, whereas the bad stuff will not be so rapidly replaced, since by and large it was only there in the first place as a result of impaired excretion/degradation.”
The Conboys don’t embrace the programmed aging perspective, but neither is their understanding of what they see the same as Aubrey’s. The way Irina explained it to me is that the age of the biological of the body is simply a measure of how much damage has accumulated, but that cycles of epigenetics and catalysis are self-reinforcing.
“Epigenetic, mRNA, and protein are steps of one process, regulation of gene expression. And none of these steps are permanent they all actively and constantly respond to cell environment — tissue and systemic milieu…With aging there is a drift which is re-calibrated by a number of rejuvenation approaches…When an auto-inductive age-elevated ligand is diluted, it cannot activate its own receptor and induce its own mRNA, so ligand levels diminish to their younger states for prolonged time.”
The Conboys theorize that these harmful proteins are part of a positive feedback loop, in other words, a cycle that is self-sustaining
epigenetic state ⇒ gene expression ⇒ translation to circulating proteins ⇒ feedback that alters the epigenetic state
With age, the body has slipped into a dysfunctional, self-sustaining cycle, and with the shock of disruption, they are able to nudge it back into a more robust and youthful cycle, also self-sustaining.
—Figure 6. Model of the dilution effect in resetting of circulatory proteome. System: A induces itself (A, red), and C (blue); A represses B (green), C represses A. A dilution of an age-elevated protein (A, at D1: initial dilution event), breaks the autoinduction and diminishes the levels of A (event 1, red arrow); the secondary target of A (B, at event 2 green arrow), then becomes de-repressed and elevated (B induces B is postulated); the attenuator of A (C, at event 3 blue arrow), has a time-delay (TD) of being diminished, as it is intracellular and was not immediately diluted, and some protein levels persist even after the lower induction of C by A. C decreases (no longer induced by A), and a re-boot of A results in the re-induction of C by A (event 4 blue arrow) leading to the secondary decrease of A signaling intensity/autoinduction, and a secondary upward wave of B (events 5 red arrow and 6 green arrow, respectively). alpha = 0.01, kc = 0.01, beta = 0.05, epsilon = 0.1, ka = 0.1. Protein removal rates from system: removalA = 0.01, removalB = 0.1, removalC = 0.01, Initial values: initialA = 1000, initialB = 400, initialC. = 700[/i]
For me, the surprising thing in Irina’s account is that there is no hysteresis in this system. The reprogramming responds to changes in the blood levels of signals within minutes. There is no homeostasis in such a system. I wonder how that can be. Life is all about homeostasis, and intuitively, we all imagine that negative feedback loops are more common than positive feedback loops. (Negative feedback loops lead to homeostasis; positive feedback loops lead to runaway, exponential change.)
Is there a clock somewhere? Is the brain special?
In the Conboy view, signals in the blood are emitted from all over the body, and not especially from the hypothalamus. If brain tissue responds in a seemingly exceptional way to proteins in the blood, it is because of selective passage of those proteins by the blood-brain barrier.
The authors remind us that in past parabiosis experiments (where blood is exchanged between old and young mice), the brain tissue of the young mice grew older but brains of the old mice didn’t get younger. This was an indication that brain aging is caused by affirmative action of “bad actors” in the plasma, and that these are able to penetrate the blood-brain barrier. This observation was part of the inspiration for the current experiments.
The corresponding procedure in humans is already FDA approved
Therapeutic Plasma Exchange (TPE) is a well-established medical procedure, and has already been performed on an experimental basis by co-author Dobri Kiprov. There is anecotal history of suggestive results, which I will write about in my next post.
Comparison with Katcher’s Elixir
This week’s announcement from the Conboys and last month’s preprint from Katcher/Horvath come from the same school of thought: that aging is coordinated through the body by signal molecules in the blood. Both demonstrated dramatic rejuvenation in rodents based on a short-term intervention, and both have plans for commercialization and human trials to begin ASAP.
So it is curious that in other ways, the programs of Katcher and Conboy are so different.
While both approaches are rooted in differing compositions of blood plasma between young and old, the Conboys focus exclusively on removing species that are inhibiting youthful regeneration, while Katcher’s approach is to add back the proteins that formerly kept the animal young.
The Conboys have fully disclosed all aspects of their experimental protocol, whereas the content of Katcher’s elixir remains a trade secret.
Katcher is on the fringe of academic research, and the Conboys’ lab is at one of the premier academic institutions in the world.
Katcher is a year further along, having experimented with different dosages and timings. Neither Katcher nor the Conboy lab has yet demonstrated life extension.
The Conboys demonstrate rejuvenation with wound healing, tissue structure, and renewal of nerve growth. Katcher’s claim is based on physiology (especially inflammation), cognitive performance, and methylation clock algorithms.
In fact, Katcher regards restoration of youthful methylation patterns as the best evidence he could offer for rejuvenation (I agree), while the Conboys are reserving judgment about the importance of methylation, and bristle at the language of a methylation “clock”.
Katcher understands the effects of plasma transfusions in terms of a broad theory (which I support). Aging is an epigenetic program, governed and enforced by a “clock” that operates via a feedback loop between circulating proteins that govern gene expression and gene expression that generate those proteins. The Conboys recognize they are working this feedback loop (their Fig 6) but they resist the theory that it is the essential cause of aging.
My guess is that a combination of their two approaches will be necessary for full remediation of aging, and that a combination of their resources, credibility, theoretical foundations, and contacts would be a transformative event for medical science, for biotech industry, and for biological theory. It is my fervent hope that Katcher and the Conboys might work together.
https://joshmitteldorf.scienceblog.com/ ... old-blood/
Re: Factor X have we finally found the fountain of Youth?
I believe that invovo cellular reprogramming is the actual mechanism of rejuveation in both of these "blood factor" experiments. I see this as a crude method of inducing such reprogramming. Better methods of cellular reprogramming will naturally have more dramatic effect.